
Small animals
The PiezoWave² makes it possible to precisely treat the target area.
Designed for quiet application with painless coupling, the unit can treat diseased tissue precisely at the right penetration depth.
RANGE OF ESWT INDICATIONS
FOR SMALL ANIMALS
Musculoskeletal disorders account for a large percentage of the problems of small animals in veterinary medicine. Dog-sport activities have become very popular in recent years. But irrespective of whether the dog is involved in competitive dog-sport activities, is a working dog or simply a canine companion in daily sports activities – every discipline places its own demands on the performance of the animal’s locomotor system. ESWT can be used to treat the following indications:
- Hip dysplasia
- Spondylosis deformans
- Tendinopathies (e.g. insertion tendinopathy of the biceps brachii tendon)
- Elbow dysplasia
- Osteoarthritis
- Medial shoulder instability (rotator cuff injury)
- Poorly healing wounds
EXAMPLES OF APPLICATIONS

HIP DYSPLASIA:
Recommended therapy source:
F7G3
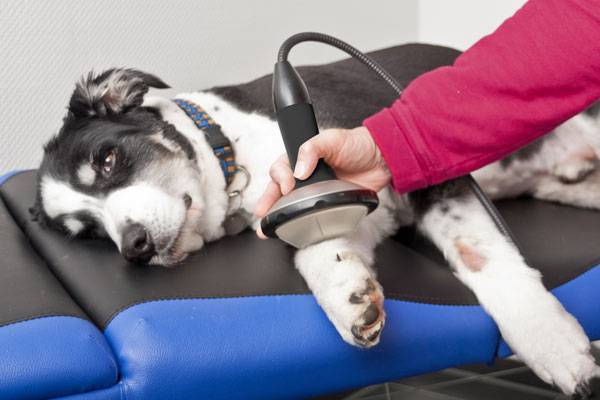
OSTEOARTHRITIS OF THE CARPAL JOINTS:
Recommended therapy source:
F7G3

POORLY HEALING WOUNDS:
Recommended therapy sources:
FBL10x5G2, F7G3

HIP DYSPLASIA
Recommended therapy source:
F7G3

OSTEOARTHRITIS OF THE CARPAL JOINTS
Recommended therapy source:
F7G3

POORLY HEALING WOUNDS
Recommended therapy sources: FBL10x5G2, F7G3
CONFIGURATION EXAMPLE:


PIEZOWAVE² VET SYSTEM
FOR SMALL ANIMALS
Convenient and effective
The F7G3 piezo therapy source is a particularly convenient therapy source with a very precise focus and a penetration depth of up to 30 mm (in the focal center). This makes it well suited to treat orthopedic disorders in small animal medicine.
The system can be expanded at any time by purchasing additional therapy sources.
A choice of foot or hand activation is available
Learn more about the therapy sources, their penetration depths, and what everything means ›
STANDARD ACCESSORIES
- Therapy source/handpiece F7G3 30 mm
- Gel pads
- 1 bottle ESWT contact gel, 250 ml
- Foot switch
- Unit trolley
The tablet computer is not included with the unit. The trigger point App is available from the App store.
TECHNICAL SPECIFICATIONS
- Power supply 220-240 VAC / 50 – 60 Hz
- Max. noise level 65 dB(A)
- Classification Class IIb (93/42 EEC)
TECHNICAL SPECIFICATIONS OF THE THERAPY SOURCE
- Energy flux density 0,018–0,403 mJ/mm²
- Intensity 20 intensity settings
- Penetration depth up to 30 mm (in the focal center)
- Penetration depth up to 40 mm (in the 5MPa distal zone)
Service
The unit requires very little servicing. To ensure its maximum life span, we recommend the following:
Control unit
An annual safety inspection is mandatory in all EU member states. The inspection should be combined with a functional check by a trained engineer.
Therapy source
The guaranteed working life of the therapy source is 5 million shockwave pulses and/or 2 years. An inspection of the therapy source by a trained engineer is recommended at the end of this period.
ELvation Medical Worldwide − Region Selector

You are visiting the website of the following region:
![]() Rest of World (ex. USA)
Rest of World (ex. USA)
Please confirm if this is your desired region to ensure we provide you with the most relevant information. You can change your region selection anytime via the menu.
The information given on this website is valid for the area of the European Union (CE area) and is intended for healthcare professionals only: it may contain information on products and indications that may not be available in all countries.
Is this not the region you were looking for? Select the right region for you:
